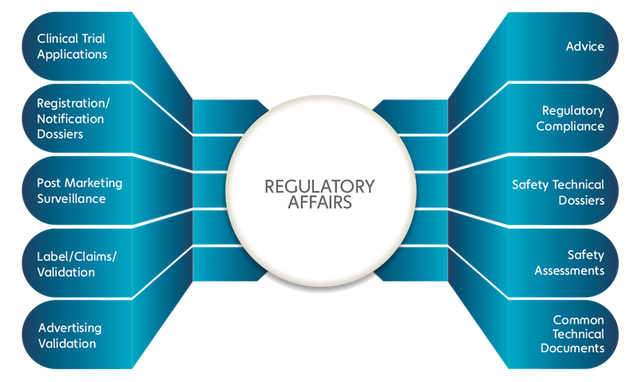
Medical Device Services Portfolio
Katalyst HealthCares & Life Sciences offers a comprehensive medical device services portfolio supporting clinical development, regulatory compliance, and post-marketing requirements. These services are designed to assist medical device manufacturers across different stages of the product lifecycle, from clinical investigations to regulatory approvals and ongoing compliance.
- Medical Device Clinical Investigational Studies
- Feasibility Studies
- Pilot Studies
- PASS Studies
- Clinical Follow-up Performance Studies
- Real-World Evidence Studies
- PMCF Studies
- Regulatory Submissions
- Product Registration
- Import/Export
- Investigational Device Exemption (IDE)
USFDA 510 (k) Submissions
Katalyst provides regulatory support for U.S. FDA submissions and related approvals required for medical device clearance and manufacturing authorization. These services help ensure compliance with applicable regulatory pathways and submission requirements.
- De-novo 510 (k) Submissions
- PMA Submissions
- Manufacturing License
- Loan Manufacturing License Approvals
- Medical and Scientific Writing Services
- CER (Clinical Evaluation Report) Writing
- Literature review summaries on Analytes
- PER Performance Evaluation Reports
- PMCF Post-marketing Clinical follow-up plans and reports for Class IIa, IIb, III Devices
- Device Vigilance & PSUR Writing
- Toxicology Reports Writing
- Audits and QMS Services (ISO13485:2016)

EU-MDR and IVDR Consulting Support
Katalyst supports medical device manufacturers in meeting European regulatory requirements under EU-MDR and IVDR, including compliance with post-market surveillance, labeling, and safety reporting obligations throughout the device lifecycle.
- Artwork & Label Management
- EU PMS FSCA Reporting
End-to-End device lifecycle support
Katalyst HealthCares & Life Sciences supports medical device manufacturers across the entire product lifecycle, from early clinical investigations through regulatory approvals and post-market activities. Our approach focuses on integrating clinical evidence generation, regulatory submissions, quality management systems, and post-marketing requirements to ensure alignment at every stage of development. By maintaining continuity across clinical, regulatory, and quality functions, we help organizations manage evolving regulatory expectations while supporting effective lifecycle planning, documentation consistency, and long-term compliance throughout device development and commercialization.
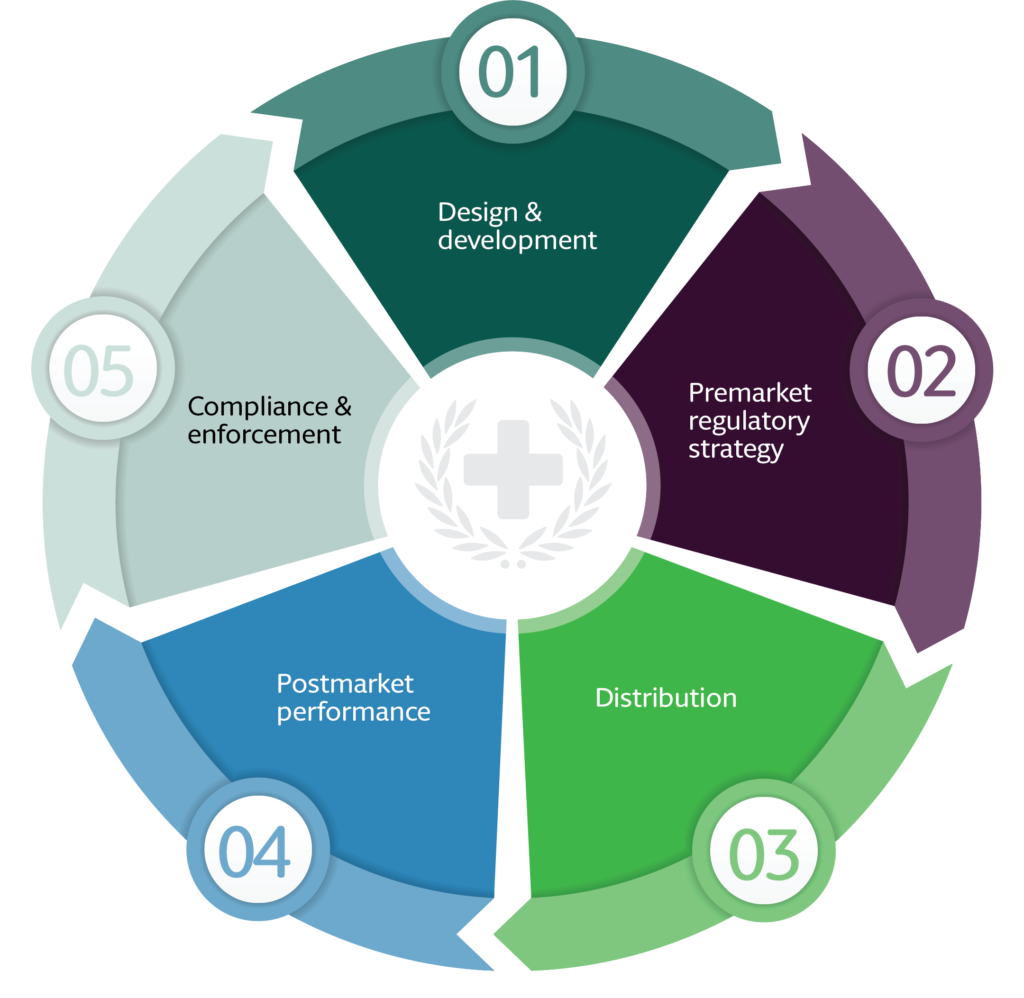
Regulatory compliance & global readiness
Our medical device services are designed to support compliance with applicable global regulatory requirements, including U.S. FDA and European regulations. Katalyst works closely with manufacturers to ensure that regulatory documentation, quality systems, and post-market obligations are addressed in a structured and compliant manner. Through ongoing regulatory support, quality oversight, and post-market regulatory activities, we assist organizations in maintaining regulatory readiness, responding to regulatory changes, and supporting sustainable market access across multiple regions.